Chlorophyll b
In this article, we will explore Chlorophyll b from different perspectives, analyzing its importance in various contexts and its impact on today's society. Chlorophyll b is a topic/element/person that has captured the attention of different sectors, generating debate and reflection around its relevance today. Throughout this article, we will examine key aspects related to Chlorophyll b, highlighting its influence in different areas and its future projection. Through a detailed and critical analysis, we will seek to delve into the complexity of Chlorophyll b, providing the reader with a complete and multidimensional vision of the topic. Join us on this journey to discover the true essence of Chlorophyll b and its impact on our contemporary society!
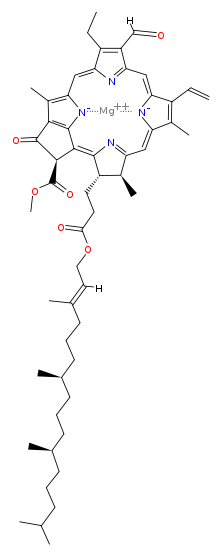
| |
| Names | |
|---|---|
| IUPAC name
Chlorophyll b
| |
| Systematic IUPAC name
Magnesium oxy}propyl)-9-vinyl-21-phorbinecarboxylatato(2-)-κ2N,N′] | |
| Other names
β-Chlorophyll
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.522 |
| EC Number |
|
| E number | E140 (colours) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C55H70MgN4O6 | |
| Molar mass | 907.492 g·mol−1 |
| Appearance | Green |
| Odor | Odorless |
| Melting point | ~ 125 °C (257 °F; 398 K) |
| Insoluble | |
| Solubility | Very soluble in ethanol, ether, pyridine Soluble in methanol |
| Absorbance | See text |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |

Chlorophyll b is a form of chlorophyll. Chlorophyll b helps in photosynthesis by absorbing light energy. It is more soluble than chlorophyll a in polar solvents because of its carbonyl group. Its color is green, and it primarily absorbs blue light.
In land plants, the light-harvesting antennae around photosystem II contain the majority of chlorophyll b. Hence, in shade-adapted chloroplasts, which have an increased ratio of photosystem II to photosystem I, there is a higher ratio of chlorophyll b to chlorophyll a. This is adaptive, as increasing chlorophyll b increases the range of wavelengths absorbed by the shade chloroplasts.
 |

|
| Structure of chlorophyll b molecule showing the long hydrocarbon tail | |
Biosynthesis
The Chlorophyll b biosynthetic pathway utilizes a variety of enzymes. In most plants, chlorophyll is derived from glutamate and is synthesised along a branched pathway that is shared with heme and siroheme. The initial steps incorporate glutamic acid into 5-aminolevulinic acid (ALA); two molecules of ALA are then reduced to porphobilinogen (PBG), and four molecules of PBG are coupled, forming protoporphyrin IX.
Chlorophyll synthase is the enzyme that completes the biosynthesis of chlorophyll b by catalysing the reaction EC 2.5.1.62
- chlorophyllide b + phytyl diphosphate chlorophyll b + diphosphate
This forms an ester of the carboxylic acid group in chlorophyllide b with the 20-carbon diterpene alcohol phytol.
References
- ^ a b c Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ "Photosynthesis pigments". Archived from the original on 2012-09-05. Retrieved 2011-01-13.
- ^ Kitajima, Kaoru; Hogan, Kevin P (2003). "Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light". Plant, Cell & Environment. 26 (6): 857–865. doi:10.1046/j.1365-3040.2003.01017.x. PMID 12803613.
- ^ Suzuki JY, Bollivar DW, Bauer CE (1997). "Genetic analysis of chlorophyll biosynthesis". Annual Review of Genetics. 31 (1): 61–89. doi:10.1146/annurev.genet.31.1.61. PMID 9442890.
- ^ Battersby, A. R. (2000). "Tetrapyrroles: the Pigments of Life. A Millennium review". Nat. Prod. Rep. 17 (6): 507–526. doi:10.1039/B002635M. PMID 11152419.
- ^ Akhtar, M. (2007). "The Modification of Acetate and Propionate Side Chains During the Biosynthesis of Haem and Chlorophylls: Mechanistic and Stereochemical Studies". Ciba Foundation Symposium 180 - the Biosynthesis of the Tetrapyrrole Pigments. Novartis Foundation Symposia. Vol. 180. pp. 131–155. doi:10.1002/9780470514535.ch8. ISBN 9780470514535. PMID 7842850.
- ^ Willows, Robert D. (2003). "Biosynthesis of chlorophylls from protoporphyrin IX". Natural Product Reports. 20 (6): 327–341. doi:10.1039/B110549N. PMID 12828371.
- ^ Schmid, H. C.; Rassadina, V.; Oster, U.; Schoch, S.; Rüdiger, W. (2002). "Pre-Loading of Chlorophyll Synthase with Tetraprenyl Diphosphate is an Obligatory Step in Chlorophyll Biosynthesis" (PDF). Biological Chemistry. 383 (11): 1769–78. doi:10.1515/BC.2002.198. PMID 12530542. S2CID 3099209.
- ^ Eckhardt, Ulrich; Grimm, Bernhard; Hortensteiner, Stefan (2004). "Recent advances in chlorophyll biosynthesis and breakdown in higher plants". Plant Molecular Biology. 56 (1): 1–14. doi:10.1007/s11103-004-2331-3. PMID 15604725. S2CID 21174896.
- ^ Bollivar, David W. (2007). "Recent advances in chlorophyll biosynthesis". Photosynthesis Research. 90 (2): 173–194. doi:10.1007/s11120-006-9076-6. PMID 17370354. S2CID 23808539.
