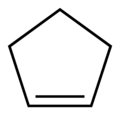
Cyclopentene
This article will address Cyclopentene from a broad and deep perspective, with the aim of providing the reader with a complete and detailed vision of this topic. The importance of Cyclopentene in today's society is undeniable, so it is essential to delve into its meaning, origin, development and repercussions. Through an exhaustive and rigorous analysis, the aim is to shed light on the different aspects that revolve around Cyclopentene, providing relevant and updated information that allows us to understand its relevance today. Likewise, different points of view and opinions of experts on the subject will be explored, in order to enrich the debate and offer a plural and enriching vision about Cyclopentene.
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclopentene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.030 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H8 | |||
| Molar mass | 68.11 g/mol | ||
| Density | 0.771 g/cm3 | ||
| Melting point | −135 °C (−211 °F; 138 K) | ||
| Boiling point | 44 to 46 °C (111 to 115 °F; 317 to 319 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −29 °C (−20 °F; 244 K) | ||
| Related compounds | |||
Related compounds
|
Cyclopentadiene Cyclobutene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Cyclopentene is a chemical compound with the formula (CH2)3(CH)2. It is a colorless liquid with a petrol-like odor. It has few applications, and thus is mainly used as a minor component of gasoline, present in concentrations of less than 1%.[1][2] It is one of the principal cycloalkenes.
History and synthesis
Cyclopentene was first prepared by Carl Gärtner in 1893 from iodocyclopentane with potassium hydroxide. He named it pentamethenylene (German: Pentamethenylen).[3]
Cyclopentene is produced industrially in large amounts by steam cracking of naphtha. In the laboratory, it is prepared by dehydration of cyclopentanol.[4] Substituted cyclopentenes are the product of the vinylcyclopropane-cyclopentene rearrangement.[5]
It can also be produced by the catalytic hydrogenation of cyclopentadiene.[6]
Reactions
The polymerization of cyclopentene by Ziegler-Natta catalysts yields 1,3-linkages, not the more typical 1,2-linked polymer.[7]
Palladium-catalyzed hydrocarboxylation of cyclopentene gives cyclopentanecarboxylic acid:[8]
- C5H8 + CO + H2O → C5H9CO2H
References
- ^ Dieter Hönicke; Ringo Födisch; Peter Claus; Michael Olson (2002). "Cyclopentadiene and Cyclopentene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_227. ISBN 978-3-527-30673-2.
- ^ "Hydrocarbon Composition of Gasoline Vapor Emissions from Enclosed Fuel Tanks". nepis.epa.gov. United States Environmental Protection Agency. 2011.
- ^ Gärtner, Carl (January 1893). "Das Pentamethenylen und sein Dibromür". Justus Liebigs Annalen der Chemie. 275 (2–3): 331–332. doi:10.1002/jlac.18932750217. ISSN 0075-4617.
- ^ B. B. Corson, V. N. Ipatieff (1939). "Cyclohexylbenzene". Organic Syntheses. 19: 36. doi:10.15227/orgsyn.019.0036.
- ^ Baldwin, John E. (2003). "Thermal Rearrangements of Vinylcyclopropanes to Cyclopentenes". Chemical Reviews. 103 (4): 1197–212. doi:10.1021/cr010020z. PMID 12683781.
- ^ D. Hönicke, R. Födisch, P. Claus, M. Olson: Cyclopentadiene and Cyclopentene, in: Ullmanns Enzyklopädie der Technischen Chemie 2002, Wiley-VCH, Weinheim.
- ^ Collins, Scott; Kelly, W. Mark (1992). "The microstructure of poly(cyclopentene) produced by polymerization of cyclopentene with homogeneous Ziegler-Natta catalysts". Macromolecules. 25 (1): 233–7. Bibcode:1992MaMol..25..233C. doi:10.1021/ma00027a039.
- ^ Sang, Rui; Kucmierczyk, Peter; Dühren, Ricarda; Razzaq, Rauf; Dong, Kaiwu; Liu, Jie; Franke, Robert; Jackstell, Ralf; Beller, Matthias (2019). "Synthesis of Carboxylic Acids by Palladium‐Catalyzed Hydroxycarbonylation". Angewandte Chemie International Edition. 58 (40): 14365–14373. doi:10.1002/anie.201908451. PMID 31390131. S2CID 199466915.
External links
 Media related to Cyclopentene at Wikimedia Commons
Media related to Cyclopentene at Wikimedia Commons