Enolate
In today's world, Enolate is a topic of constant interest and covers a wide range of aspects. From its influence on society to its implications on the global economy, Enolate has become a focal point in everyday conversations. With an impact that transcends borders and cultures, Enolate has positioned itself as a relevant and constantly evolving topic. In this article, we will explore different perspectives and approaches related to Enolate, with the aim of understanding its importance in the current context and its projection for the future.

In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl (RR'C=O) compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.[1][2][3][4]
Bonding and structure

Enolate anions are electronically related to allyl anions. The anionic charge is delocalized over the oxygen and the two carbon sites. Thus they have the character of both an alkoxide and a carbanion.[5]

Although enolate salts are often drawn as simple ion pairs, in fact they adopt complicated structures often featuring aggregates.[7]
Preparation
Carbonyl compounds with an α hydrogen atom deprotonate to give enolates:[8][9]

Base mediates the process, but Lewis acidity plays a key role in stabilizing the product. Often the (weak) Lewis acid is simply the alkali counterion to an Arrhenius base (1.); unsurprisingly, reactivity with such salts varies from lithium to cesium. Alternatively, enolates can be generated from a molecular Lewis acid and a weak Brønsted base (a frustrated Lewis pair; 2.):

Most substrates have multiple α hydrogen atoms, and in principle could give multiple enolate isomers. For example, with methylcyclohexanone:

However, reaction conditions can control both the resulting enolate's regio-[11] and stereochemistry.[12] This provides one of the best understood synthetic strategies to introduce chemical complexity in total syntheses.[citation needed]
Alternatively, an enone can serve as a protecting group, masking a specific enol.[13] Reaction with a hydride or dissolving-metal reduction then forms an enol, as in this total synthesis of progesterone:[14]

Regiochemistry
The kinetic-versus-thermodynamic distinction is key to regiocontrol during deprotonation. Substitution improves alkene thermodynamics through additional hyperconjugation, but hinders initial proton loss. In the methylcyclohexanone example above, the trisubstituted enolate deprotonates more quickly: it is the kinetic enolate. The tetrasubstituted enolate is more stable, and dominant in thermodynamic equilibrium.
Base strength determines the regioisomeric ratio. With strong bases and weak Lewis acids, deprotonation is quantitative and irreversible, trapping the kinetic enolate. Typically kinetic enolates are generated using lithium diisopropylamide (LDA), often in slight excess and at low temperature.[15] Weaker alkoxide bases and stronger Lewis acids instead reversibly deprotonate the substrate, affording thermodynamically-favored enolates.
Stereochemistry
Most enolization conditions give Z enolates from ketones and E enolates from esters, but HMPA is known to reverse the stereoselectivity of deprotonation.

Likewise different Lewis acids give different enolate geometries:[12]

The Ireland model attempts to rationalize stereoselection[16][17][18][19] with a six-membered, cyclic,[20] monomeric transition state proposal. For deprotonation to occur, an α C-H σ bond must overlap the π* orbital of the carbonyl:
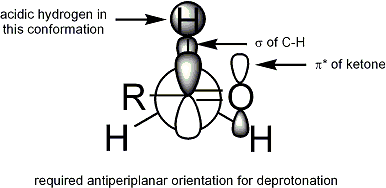
In the Ireland model, the larger substituent on the electrophile (for the ester above, methyl) adopts an equatorial disposition in the transition state, leading to a preference for E enolates.

The Ireland model fails often. It is not known when, if ever, the intermediates are monomeric and cyclic like the model proposes.
Reactions
Powerful nucleophiles, enolates react with a variety of electrophiles at oxygen and carbon. Controlling which atom enolates react at has drawn much attention. Reaction at carbon is thermodynamically favored. Kinetically, the negative charge in enolates is concentrated on the oxygen. However, the oxygen center is also highly solvated, which can lead to alkylation at carbon.[21]
Reaction at oxygen traps the enolate as a (silyl)[22] enol ether[23] or ester.[24] Such species can be carried through other transformations relatively inertly, but then are released in the presence of Lewis acids (the Mukaiyama aldol reaction):
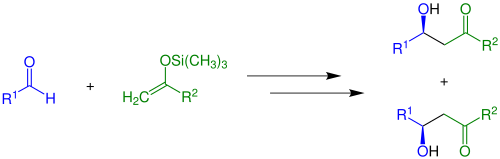
Other important electrophiles are aldehydes/ketones (the aldol reaction) and Michael acceptors.[25]
See also
References
- ^ Stolz, Daniel; Kazmaier, Uli (2010). "Metal Enolates as Synthons in Organic Chemistry". PATai's Chemistry of Functional Groups. doi:10.1002/9780470682531.pat0423. ISBN 978-0-470-68253-1.
- ^ Hart, David J.; Ha, Deok Chan (1989). "The ester enolate-imine condensation route to .beta.-lactams". Chemical Reviews. 89 (7): 1447–1465. doi:10.1021/cr00097a003.
- ^ Wu, George; Huang, Mingsheng (2006). "Organolithium Reagents in Pharmaceutical Asymmetric Processes". Chemical Reviews. 106 (7): 2596–2616. doi:10.1021/cr040694k. PMID 16836294.
- ^ Curti, Claudio; Battistini, Lucia; Sartori, Andrea; Zanardi, Franca (2020). "New Developments of the Principle of Vinylogy as Applied to π-Extended Enolate-Type Donor Systems". Chemical Reviews. 120 (5): 2448–2612. doi:10.1021/acs.chemrev.9b00481. PMC 7993750. PMID 32040305.
- ^ IUPAC, Compendium of Chemical Terminology, 5th ed. (the "Gold Book") (2025). Online version: (2006–) "Enolates". doi:10.1351/goldbook.E02123
- ^ Nichols, Michael A.; Leposa, Christina M.; Hunter, Allen D.; Zeller, Matthias (2007). "Crystal Structures of Hexameric and Dimeric Complexes of Lithioisobutyrophenone". Journal of Chemical Crystallography. 37 (12): 825–829. doi:10.1007/s10870-007-9255-0. S2CID 97183362.
- ^ Reich, Hans J. (2013). "Role of Organolithium Aggregates and Mixed Aggregates in Organolithium Mechanisms". Chemical Reviews. 113 (9): 7130–7178. doi:10.1021/cr400187u. PMID 23941648.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ Manfred Braun (2015). Modern Enolate Chemistry: From Preparation to Applications in Asymmetric Synthesis. Wiley‐VCH. doi:10.1002/9783527671069. ISBN 978-3-527-67106-9.
- ^ Kong, Jianshe; Meng, Tao; Ting, Pauline; Wong, Jesse (2010). "Preparation of Ethyl 1-Benzyl-4-Fluoropiperidine-4-Carboxylate". Organic Syntheses. 87: 137. doi:10.15227/orgsyn.087.0137.
- ^ Gall, Martin; House, Herbert O. (1972). "The Formation and Alkylation of Specific Enolate Anions from an Unsymmetrical Ketone: 2-Benzyl-2-methylcyclohexanone and 2-Benzyl-6-methylcyclohexanone". Org. Synth. 52: 39. doi:10.15227/orgsyn.052.0039.
- ^ a b Brown, H. C.; Dhar, R. K.; Bakshi, R. K.; Pandiarajan, P. K.; Singaram, B. (1989). "Major effect of the leaving group in dialkylboron chlorides and triflates in controlling the stereospecific conversion of ketones into either E- or Z-enol borinates". Journal of the American Chemical Society. 111 (9): 3441–3442. doi:10.1021/ja00191a058.
- ^ Stork, G.; Singh, J., J. Am. Chem. Soc. 1974, 96, 6181.
- ^ Stork, G.; McMurry, J. E., J. Am. Chem. Soc. 1967, 89, 5464.
- ^ Christine Wedler; Hans Schick (1998). "Synthesis of Β-lactones By Aldolization of Ketones with Phenyl Ester Enolates: 3,3-Dimethyl-1-oxaspirononan-2-one". Org. Synth. 75: 116. doi:10.15227/orgsyn.075.0116.
- ^ Ireland, R. E.; Willard, A. K. (1975). "The stereoselective generation of ester enolates". Tetrahedron Letters. 16 (46): 3975–3978. doi:10.1016/S0040-4039(00)91213-9.
- ^ Narula, A. S. (1981). "An analysis of the diastereomeric transition state interactions for the kinetic deprotonation of acyclic carbonyl derivatives with lithium diisopropylamide". Tetrahedron Letters. 22 (41): 4119–4122. doi:10.1016/S0040-4039(01)82081-5.
- ^ Ireland, RE; Wipf, P; Armstrong, JD (1991). "Stereochemical control in the ester enolate Claisen rearrangement. 1. Stereoselectivity in silyl ketene acetal formation". Journal of Organic Chemistry. 56 (2): 650–657. doi:10.1021/jo00002a030.
- ^ Xie, L; Isenberger, KM; Held, G; Dahl, LM (October 1997). "Highly Stereoselective Kinetic Enolate Formation: Steric vs Electronic Effects". Journal of Organic Chemistry. 62 (21): 7516–7519. doi:10.1021/jo971260a. PMID 11671880.
- ^ Directed Aldol Synthesis – Formation of E-enolate and Z-enolate
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 551, ISBN 978-0-471-72091-1
- ^ Mukaiyama, T.; Kobayashi, S. Org. React. 1994, 46, 1. doi:10.1002/0471264180.or046.01
- ^ Mukaiyama, Teruaki; Kobayashi, Shū (1994). "Tin(II) Enolates in the Aldol, Michael, and Related Reactions". Organic Reactions. pp. 1–103. doi:10.1002/0471264180.or046.01. ISBN 0-471-26418-0.
- ^ G. Roscher (2007). "Vinyl Esters". Ullmann's Encyclopedia of Chemical Technology. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_419. ISBN 978-3527306732. S2CID 241676899.
- ^ Seebach, Dieter (1988). "Structure and Reactivity of Lithium Enolates. From Pinacolone to SelectiveC-Alkylations of Peptides. Difficulties and Opportunities Afforded by Complex Structures". Angewandte Chemie International Edition in English. 27 (12): 1624–1654. doi:10.1002/anie.198816241.

