Iridium(IV) oxide
In this article we will be addressing Iridium(IV) oxide, a topic that has captured the attention of numerous individuals around the world. In order to provide a comprehensive and detailed view on Iridium(IV) oxide, we will explore different aspects related to this topic, from its origins to its relevance today. Additionally, we will examine various perspectives and opinions of experts in the field, with the purpose of offering readers a deep and complete understanding of Iridium(IV) oxide. In addition, we will analyze the impact that Iridium(IV) oxide has had in different areas, as well as its possible implications for the future. Ultimately, this article aims to shed light on Iridium(IV) oxide, providing readers with an informed and enriching perspective on this topic.
 | |
| Names | |
|---|---|
| Other names
Iridium dioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.572 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| IrO2 | |
| Molar mass | 224.22 g/mol |
| Appearance | blue-black solid |
| Density | 11.66 g/cm3 |
| Melting point | 1,100 °C (2,010 °F; 1,370 K) decomposes |
| insoluble | |
| +224.0·10−6 cm3/mol | |
| Structure | |
| Rutile (tetragonal) | |
| Octahedral (Ir); Trigonal (O) | |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
iridium(IV) fluoride, iridium disulfide |
Other cations
|
rhodium dioxide, osmium dioxide, platinum dioxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Iridium(IV) oxide, IrO2, is the only well-characterised oxide of iridium. It is a blue-black solid, used with other rare oxides to coat anodes.
Synthesis
As described by its discoverers, it can be formed by treating the green form of iridium trichloride with oxygen at high temperatures:
- 2 IrCl3 + 2 O2 → 2 IrO2 + 3 Cl2
A hydrated form is also known.[1]
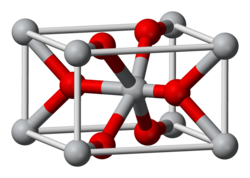
Structure
The compound adopts the TiO2 rutile structure, featuring six coordinate iridium and three coordinate oxygen.[2] It forms a tetragonal lattice with lattice parameters of 4.5Å and 3.15Å.[3]
Mechanical properties
Oxide materials are typically hard and brittle.[4] Indeed, iridium oxide does not easily deform under stress,[5] instead cracking easily.[6] Measured deflections of a thin, cantilevered iridium oxide film indicate a Young’s modulus of 300 ± 15 GPa,[5] substantially lower than the Young's modulus of metallic iridium (517 GPa).[7]
Applications
Iridium dioxide can be used to make coated electrodes[8] for industrial electrolysis or as microelectrodes for electrophysiology.[9] In electrolytic applications, IrO2 films evolve O2 efficiently.[10]
Electrode manufacture typically requires high-temperature annealing.[11]
Fracture and delamination are well-known problems when fabricating devices that incorporate iridium oxide film. One cause of delamination is lattice mismatch between iridium oxide and the substrate. Sputtering iridium oxide on a liquid crystal polymer has been proposed to avoid mismatch,[12] but sputtered films spontaneously delaminate during cyclic voltammetry if the maximum potential bias exceeds 0.9 V.[13]
References
- ^ H. L. Grube (1963). "The Platinum Metals". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. NY: Academic Press. p. 1590.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ Bestaoui, N.; Prouzet, E.; Deniard, P.; Brec, R. (1993). "Structural and analytical characterization of an iridium oxide thin layer". Thin Solid Films. 235 (1–2): 35–42. Bibcode:1993TSF...235...35B. doi:10.1016/0040-6090(93)90239-l. ISSN 0040-6090.
- ^ Dillingham, Giles; Roberts, Rose (2023). Advances in Structural Adhesive Bonding (Second Edition) (2nd ed.). Woodhead Publishing. pp. 289–325. ISBN 9780323984379.
- ^ a b Rivas, Manuel; Rudy, Ryan Q.; Sanchez, Bradley; Graziano, Milena B.; Fox, Glen R.; Sunal, Paul; Nataraj, Latha; Sandoz-Rosado, Emil; Leff, Asher C.; Huey, Bryan D.; Polcawich, Ronald G.; Hanrahan, Brendan (2020-08-01). "Iridium oxide top electrodes for piezo- and pyroelectric performance enhancements in lead zirconate titanate thin-film devices". Journal of Materials Science. 55 (24): 10351–10363. Bibcode:2020JMatS..5510351R. doi:10.1007/s10853-020-04766-5. ISSN 1573-4803.
- ^ Mailley, S.C; Hyland, M; Mailley, P; McLaughlin, J.M; McAdams, E.T (2002). "Electrochemical and structural characterizations of electrodeposited iridium oxide thin-film electrodes applied to neurostimulating electrical signal". Materials Science and Engineering: C. 21 (1–2): 167–175. doi:10.1016/s0928-4931(02)00098-x. ISSN 0928-4931.
- ^ "Young's Modulus, Tensile Strength and Yield Strength Values for some Materials". www.engineeringtoolbox.com. Retrieved 2024-05-12.
- ^ "改性二氧化铱电极研制--《无机盐工业》1998年03期". www.cnki.com.cn. Retrieved 2021-05-21.
- ^ Cogan, Stuart F. (August 2008). "Neural Stimulation and Recording Electrodes". Annual Review of Biomedical Engineering. 10 (1): 275–309. doi:10.1146/annurev.bioeng.10.061807.160518. ISSN 1523-9829. PMID 18429704.
- ^ Wendt, Hartmut; Kolb, Dieter M.; Engelmann, Gerald E.; Ziegler, Jörg C. (15 Oct 2011). "Electrochemistry, 1: Fundamentals". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_183.pub4. ISBN 978-3-527-30673-2.
- ^ Dong, Qiuchen; Song, Donghui; Huang, Yikun; Xu, Zhiheng; Chapman, James H.; Willis, William S.; Li, Baikun; Lei, Yu (2018). "High-temperature annealing enabled iridium oxide nanofibers for both non-enzymatic glucose and solid-state pH sensing". Electrochimica Acta. 281: 117–126. doi:10.1016/j.electacta.2018.04.205. ISSN 0013-4686.
- ^ Wang, K.; Chung-Chiun Liu; Durand, D.M. (2009). "Flexible Nerve Stimulation Electrode With Iridium Oxide Sputtered on Liquid Crystal Polymer". IEEE Transactions on Biomedical Engineering. 56 (1): 6–14. Bibcode:2009ITBE...56....6W. doi:10.1109/TBME.2008.926691. ISSN 0018-9294. PMC 2738844. PMID 19224713.
- ^ Cogan, Stuart F.; Ehrlich, Julia; Plante, Timothy D.; Smirnov, Anton; Shire, Douglas B.; Gingerich, Marcus; Rizzo, Joseph F. (2009). "Sputtered iridium oxide films for neural stimulation electrodes". Journal of Biomedical Materials Research Part B: Applied Biomaterials. 89B (2): 353–361. doi:10.1002/jbm.b.31223. ISSN 1552-4973. PMC 7442142. PMID 18837458.
