Iron(II) phosphate
In this article, we will explore the impact of Iron(II) phosphate on different aspects of contemporary society. From its influence on the economy to its relevance in the field of health, Iron(II) phosphate has played a fundamental role in shaping our world today. Through a comprehensive analysis, we will examine how Iron(II) phosphate has shaped our perceptions, behaviors and decisions, as well as its future projection. With this comprehensive approach, we aim to shed light on the complexity and scope of Iron(II) phosphate, giving voice to diverse perspectives and enriching the debate around this topic of global resonance.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Iron(II) phosphate
| |
| Other names
Ferrous phosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.035.456 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Fe3(PO4)2 | |
| Appearance | brown powder |
| Density | 2.61 g/cm3 (octahydrate) |
| Melting point | 180 °C (356 °F; 453 K) (octahydrate) decomposes[1] |
| insoluble | |
| Structure | |
| monoclinic (octahydrate) | |
| C 2/m | |
a = 10.086 (octahydrate), b = 13.441 (octahydrate), c = 4.703 (octahydrate) α = 90°, β = 104.27°, γ = 90°
| |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P280, P304+P340, P305+P351+P338, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
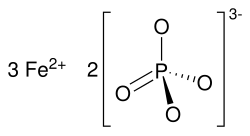
Iron(II) phosphate, also ferrous phosphate,[3] Fe3(PO4)2, is an iron salt of phosphoric acid.
Natural occurrences
The mineral vivianite is a naturally occurring form of hydrated iron(II) phosphate.
Production
It can be formed by the reaction of ferrous hydroxide with phosphoric acid to produce hydrated iron(II) phosphate.
See also
References
- ^ "iron(II) phosphate octahydrate". chemister.ru. Retrieved 2 July 2014.
- ^ "Safety Data Sheet". fishersci.com. Retrieved 12 August 2023.
- ^ "Iron(II) Phosphate". EndMemo.com. Archived from the original on 24 January 2016. Retrieved 22 January 2016.
External links
![]() Media related to Iron(II) phosphate at Wikimedia Commons
Media related to Iron(II) phosphate at Wikimedia Commons

