Peroxynitrous acid
Today, Peroxynitrous acid is a topic of great relevance and interest to a large sector of the population. This issue has captured the attention of experts, scholars and professionals from different fields, who have dedicated their time and effort to analyzing it from various approaches. Furthermore, Peroxynitrous acid has generated a debate in society, giving rise to conflicting opinions and divergent positions. Given this situation, it is relevant to deepen our knowledge of Peroxynitrous acid and explore its implications in different contexts. For this purpose, this article will address Peroxynitrous acid in a detailed and critical manner, in order to offer a comprehensive vision of this current topic.
This article needs additional citations for verification. (January 2007) |
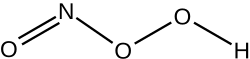 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Peroxynitrous acid[citation needed] | |
| Systematic IUPAC name | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| 49207 | |
| MeSH | Peroxynitrous+Acid |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NHO 3 | |
| Molar mass | 63.0128 g mol−1 |
| Conjugate base | Peroxynitrite |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Peroxynitrous acid (HNO3) is a reactive nitrogen species (RNS). It is the conjugate acid of peroxynitrite (ONOO−). It has a pKa of approximately 6.8. It is formed in vivo from the diffusion-controlled reaction of nitrogen monoxide (ON•) and superoxide (O•−
2). It is an isomer of nitric acid and isomerises with a rate constant of k = 1.2 s−1, a process whereby up to 5% of hydroxyl and nitrogen dioxide radicals may be formed. It oxidises and nitrates aromatic compounds in low yield. The mechanism may involve a complex between the aromatic compound and ONOOH, and a transition from the cis- to the trans-configuration of ONOOH.[3] Peroxynitrous acid is also important in atmospheric chemistry.
References
- ^ N.Connelly and T. Damhus, IUPAC. Nomenclature of Inorganic Chemistry, RSC Publishing, Cambridge, 2005
- ^ "Peroxynitrous Acid - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 11 April 2012.
- ^ Koppenol, W. H.; Bounds, P. L.; Nauser, T.; Kissner, R.; Rüegger, H. (2012). "Peroxynitrous acid: controversy and consensus surrounding an enigmatic oxidant". Dalton Transactions. 41: 13779–13787.
