Potassium pyrosulfate
Today, Potassium pyrosulfate is a highly relevant topic that has captured the attention of people of all ages and interests. With a significant impact on different aspects of daily life, Potassium pyrosulfate has generated debates, controversy and great interest at a global level. From its origins to its influence today, Potassium pyrosulfate has left an imposing mark on society, culture and history. In this article, we will explore different facets of Potassium pyrosulfate, from its origins to its impact today, analyzing its importance and relevance in different contexts.
 | |
| Names | |
|---|---|
| IUPAC name
dipotassium (sulfonatooxy)sulfonate
| |
| Other names
Potassium pyrosulphate; potassium disulfate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.288 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| K2O7S2 | |
| Molar mass | 254.31 g·mol−1 |
| Density | 2.28 g/cm3 |
| Melting point | 325 °C (617 °F; 598 K) |
| 25.4 g/100 mL (20 °C) | |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H314, H331 | |
| P260, P261, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P321, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
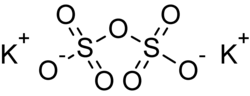
Potassium pyrosulfate, or potassium disulfate, is an inorganic compound with the chemical formula K2S2O7.
Production
Potassium pyrosulfate is obtained by the thermal decomposition of other salts, most directly from potassium bisulfate:[1]
- 2 KHSO4 → K2S2O7 + H2O
Temperatures above 600°C further decompose potassium pyrosulfate to potassium sulfate and sulfur trioxide however:[2]
- K2S2O7 → K2SO4 + SO3
Other salts, such as potassium trisulfate,[3] can also decompose into potassium pyrosulfate.
Chemical structure
Potassium pyrosulfate contains the pyrosulfate anion which has a dichromate-like structure. The geometry can be visualized as a tetrahedron with two corners sharing the SO4 anion's configuration and a centrally bridged oxygen atom.[4] A semi-structural formula for the pyrosulfate anion is O3SOSO32−. The oxidation state of sulfur in this compound is +6.
Uses
Potassium pyrosulfate is used in analytical chemistry; samples are fused with potassium pyrosulfate, (or a mixture of potassium pyrosulfate and potassium fluoride) to ensure complete dissolution prior to a quantitative analysis.[5][6]
The compound is also present in a catalyst in conjunction with vanadium(V) oxide in the industrial production of sulfur trioxide.[7]
See also
References
- ^ Washington Wiley, Harvey (1895). Principles and Practice of Agricultural Analysis: Fertilizers. Easton, PA.: Chemical Publishing Co. p. 218. Retrieved 31 December 2015.
Potassium disulfate.
- ^ Iredelle Dillard Hinds, John (1908). Inorganic Chemistry: With the Elements of Physical and Theoretical Chemistry. New York: John Wiley & Sons. p. 547. Retrieved 31 December 2015.
Potassium disulfate.
- ^ Brauer, Georg (1963). Handbook of Preparative Inorganic Chemistry Vol. 2, 2nd Ed. New York: Academic Press. p. 1716. ISBN 9780323161299.
{{cite book}}: ISBN / Date incompatibility (help) - ^ Ståhl, K.; Balic-Zunic, T.; da Silva, F.; Eriksen, K. M.; Berg, R. W.; Fehrmann, R. (2005). "The crystal structure determination and refinements of K2S2O7, KNaS2O7 and Na2S2O7 from X-ray powder and single crystal diffraction data". Journal of Solid State Chemistry. 178 (5): 1697–1704. Bibcode:2005JSSCh.178.1697S. doi:10.1016/j.jssc.2005.03.022.
- ^ Trostbl, L. J.; Wynne, D. J. (1940). "Determination of quartz (free silica) in refractory clays". Journal of the American Ceramic Society. 23 (1): 18–22. doi:10.1111/j.1151-2916.1940.tb14187.x.
- ^ Sill, C. W. (1980). "Determination of gross alpha, plutonium, neptunium, and/or uranium by gross alpha counting on barium sulphate". Analytical Chemistry. 52 (9): 1452–1459. doi:10.1021/ac50059a018.
- ^ Burkhardt, Donald (1965). "Sulfur trioxide production, US3362786A". Google Patents. Retrieved 31 December 2015.
