Ammonium cyanide
In this article, we will delve into the fascinating world of Ammonium cyanide_, exploring its many facets and its relevance in today's society. Throughout history, Ammonium cyanide_ has played a fundamental role in multiple aspects of human life, from its impact on culture and art, to its influence on economics and politics. Through a detailed and exhaustive analysis, we aim to shed light on Ammonium cyanide_ and its importance in the contemporary world, offering new perspectives and approaches to understand its current relevance.
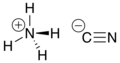 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| [NH4]CN | |
| Molar mass | 44.0559 g/mol |
| Appearance | colourless crystalline solid |
| Density | 1.02 g/cm3 |
| Melting point | 36 °C (decomp.) |
| very soluble | |
| Solubility | very soluble in alcohol |
| Structure | |
| cubic | |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ammonium cyanide is an unstable inorganic compound with the chemical formula NH4CN. It is the ammonium salt of hydrogen cyanide. It consists of ammonium cations NH+4 and cyanide anions CN−. Its structural formula is [NH4]+[C≡N]−.
Uses
Ammonium cyanide is generally used in organic synthesis.[citation needed] Being unstable, it is not shipped or sold commercially.
Preparation
Ammonium cyanide is prepared by combining solutions of hydrogen cyanide and ammonia:[citation needed]
- HCN + NH3 → NH4CN
It may be prepared by the reaction of calcium cyanide and ammonium carbonate:[citation needed]
- Ca(CN)2 + (NH4)2CO3 → 2 NH4CN + CaCO3
In dry state, ammonium cyanide is made by heating a mixture of potassium cyanide or potassium ferrocyanide with ammonium chloride and condensing the vapours into ammonium cyanide crystals:[citation needed]
- KCN + NH4Cl → NH4CN + KCl
Reactions
Ammonium cyanide decomposes to ammonia and hydrogen cyanide, often forming a black polymer of hydrogen cyanide:[1]
- NH4CN → NH3 + HCN
It undergoes salt metathesis reaction in solution with a number of metal salts to form metal–cyanide complexes.
Reaction with ketones and aldehydes yield aminonitriles, as in the first step of the Strecker amino acid synthesis:
- NH4CN + (CH3)2CO → (CH3)2C(NH2)CN + H2O
Toxicity
Ammonium cyanide is highly toxic.
Notes
- ^ Matthews, Clifford N (1991). "Hydrogen cyanide polymerization: A preferred cosmochemical pathway". Bioastronomy: The Search for Extraterrestrial Life—The Exploration Broadens. Lecture Notes in Physics. Vol. 390. pp. 85–87. doi:10.1007/3-540-54752-5_195. ISBN 978-3-540-54752-5.
References
- A. F. Wells, Structural Inorganic Chemistry, 5th ed., Oxford University Press, Oxford, UK, 1984.
