Cyclopropenone
In this article, we will explore in depth the topic of Cyclopropenone, which has been the subject of interest and debate in various areas. From its origins to its relevance today, we will address its many facets and its impact on society. Through an exhaustive and rigorous analysis, we seek to shed light on different aspects related to Cyclopropenone, providing valuable information and diverse perspectives to enrich the knowledge of our readers. By exposing data, testimonies and relevant studies, we aim to offer a complete and objective vision that allows us to understand the importance of Cyclopropenone in different contexts and situations.

| |
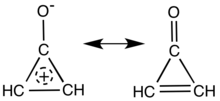 Main resonance structures of cyclopropenone.
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Cycloprop-2-en-1-one | |
| Other names
Cyclopropenone, Cyclopropene-3-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H2O | |
| Molar mass | 54.048 g·mol−1 |
| Appearance | Colorless liquid |
| Melting point | −29 to −28 °C (−20 to −18 °F; 244 to 245 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Cyclopropenone is an organic compound with molecular formula C3H2O consisting of a cyclopropene carbon framework with a ketone functional group. It is a colorless, volatile liquid that boils near room temperature. Neat cyclopropenone polymerizes upon standing at room temperature. The chemical properties of the compound are dominated by the strong polarization of the carbonyl group, which gives a partial positive charge with aromatic stabilization on the ring and a partial negative charge on oxygen. It is an aromatic compound.
See also
References
- ^ R. Breslow, J. Pecoraro, T. Sugimoto "Cyclpropenone" Org. Synth. 1977, vol. 57, pp. 41. doi:10.15227/orgsyn.057.0041
- ^ Breslow, Ronald; Oda, Masaji (1972-06-01). "Isolation and characterization of pure cyclopropenone". Journal of the American Chemical Society. 94 (13): 4787–4788. doi:10.1021/ja00768a089. ISSN 0002-7863.
- ^ "Experiments show cyclopropenone is aromatic". Chem. Eng. News. 61 (38): 33. 1983. doi:10.1021/cen-v061n038.p033.
- ^ Peart, Patricia A.; Tovar, John D. (2010). "Poly(cyclopropenone)s: Formal Inclusion of the Smallest Hückel Aromatic into π-Conjugated Polymers". J. Org. Chem. 76 (15): 5689–5696. doi:10.1021/jo101108f. PMID 20704438.
