Manganese(II,III) oxide
Currently, Manganese(II,III) oxide is a topic that has gained great relevance in various areas of society. From politics to popular culture, Manganese(II,III) oxide has become a point of constant interest and debate. Over time, interest in Manganese(II,III) oxide has increased, leading to deeper research and the generation of discussions around its importance and repercussions. In this article, we will explore different perspectives and approaches related to Manganese(II,III) oxide, in order to offer a broader and more detailed vision on this topic that is so relevant today.
| |
| Names | |
|---|---|
| IUPAC name
manganese(II) dimanganese(III) oxide
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.013.879 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Mn3O4 MnO·Mn2O3 | |
| Molar mass | 228.812 g/mol |
| Appearance | brownish-black powder |
| Density | 4.86 g/cm3 |
| Melting point | 1,567 °C (2,853 °F; 1,840 K) |
| Boiling point | 2,847 °C (5,157 °F; 3,120 K) |
| insoluble | |
| Solubility | soluble in HCl |
| +12,400·10−6 cm3/mol | |
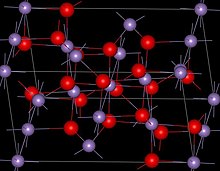
| Structure | |
| Spinel (tetragonal), tI28 | |
| I41/amd, No. 141 | |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
C 5 mg/m3 |
REL (Recommended)
|
None established |
IDLH (Immediate danger)
|
N.D. |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
149 J·mol−1·K−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−1387 kJ·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Manganese(II,III) oxide is the chemical compound with formula Mn3O4. Manganese is present in two oxidation states +2 and +3 and the formula is sometimes written as MnO·Mn2O3. Mn3O4 is found in nature as the mineral hausmannite.
Preparation
Mn3O4 formed when any manganese oxide is heated in air above 1000 °C. Considerable research has centred on producing nanocrystalline Mn3O4 and various syntheses that involve oxidation of MnII or reduction of MnVI.
Reactions
Mn3O4 has been found to act as a catalyst for a range of reactions e.g. the oxidation of methane and carbon monoxide; the decomposition of NO, the reduction of nitrobenzene and the catalytic combustion of organic compounds.
Structure
Mn3O4 has the spinel structure, where the oxide ions are cubic close packed and the MnII occupy tetrahedral sites and the MnIII octahedral sites. The structure is distorted due to the Jahn–Teller effect. At room temperature Mn3O4 is paramagnetic, below 41-43 K, it is ferrimagnetic although this has been reported as reducing in nanocrystalline samples to around 39 K.
Uses
Mn3O4 is sometimes used as a starting material in the production of soft ferrites e.g. manganese zinc ferrite, and lithium manganese oxide, used in lithium batteries.
Manganese tetroxide can also be used as a weighting agent while drilling reservoir sections in oil and gas wells.[citation needed]
References
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0381". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Hausmannite Mn3O4 nanorods: synthesis, characterization and magnetic properties Jin Du et al. Nanotechnology, (2006),17 4923-4928, doi:10.1088/0957-4484/17/19/024
- ^ One-step synthesis of Mn3O4 nanoparticles: Structural and magnetic study Vázquez-Olmos A., Redón R, Rodríguez-Gattorno G., Mata-Zamora M.E., Morales-Leal F, Fernández-Osorio A.L, Saniger J.M. Journal of Colloid and Interface Science, 291, 1, (2005), 175-180 doi:10.1016/j.jcis.2005.05.005
- ^ Use of Carbonaceous Polysaccharide Microspheres as Templates for Fabricating Metal Oxide Hollow Spheres Xiaoming Sun, Junfeng Liu, Yadong Li, Chemistry - A European Journal,(2005), 12, 7, 2039 – 2047, doi:10.1002/chem.200500660
- ^ The reduction and oxidation behaviour of manganese oxides Stobhe E.R, de Boer A.D., Geus J.W., Catalysis Today. (1999), 47, 161–167. doi:10.1016/S0920-5861(98)00296-X
- ^ An in situ XRD investigation of singly and doubly promoted manganese oxide methane coupling catalysts.Moggridge G.D, Rayment T, Lambert R.M. Journal of Catalysis, (1992), 134, 242–252, doi:10.1016/0021-9517(92)90225-7
- ^ NO Decomposition over Mn2O3 and Mn3O4. Yamashita T, Vannice A., Journal of Catalysis (1996),163, 158–168, doi:10.1006/jcat.1996.0315
- ^ Selective reduction of nitrobenzene to nitrosobenzene over different kinds of trimanganese tetroxide catalysts.Wang W.M., Yang Y.N., Zhang J.Y., Applied Catalysis A. (1995), 133, 1, 81–93 doi:10.1016/0926-860X(95)00186-7
- ^ Catalytic combustion of C3 hydrocarbons and oxygenates over Mn3O4. Baldi M, Finocchio E, Milella F, Busca G., Applied Catalysis B. (1998), 16, 1, 43–51, doi:10.1016/S0926-3373(97)00061-1
- ^ Magnetic Structure of Mn3O4 by Neutron Diffraction Boucher B., Buhl R., Perrin M., J. Appl. Phys. 42, 1615 (1971); doi:10.1063/1.1660364
- ^ Synthesis of superparamagnetic Mn3O4 nanocrystallites by ultrasonic irradiation I.K. Gopalakrishnan, N. Bagkar, R. Ganguly and S.K. Kulshreshtha Journal of Crystal Growth 280, 3-4, (2005), 436-441, doi:10.1016/j.jcrysgro.2005.03.060
- ^ Method of making manganese-zinc ferrite U.S Patent number: 4093688 (1978) Arthur Withop, Roger Emil Travagli
- ^ Process for preparing lithium manganese oxides, U.S Patent number: 6706443,(2004), Horst Krampitz, Gerhard Wohner