Diborane(4)
In today's article we are going to delve into the fascinating world of Diborane(4). From its origins to its influence on today's society, we will explore the different facets and aspects that make Diborane(4) a topic of interest to a wide spectrum of audiences. We will analyze its impact in different areas, its evolution over time and the possible implications it has for the future. Join us on this journey of discovery and learning about Diborane(4), where we will seek to shed light on its most relevant aspects and delve into its meaning in the contemporary world.
| |
| Names | |
|---|---|
| IUPAC name
Diborane(4)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| 24760 | |
PubChem CID
|
|
| |
| |
| Properties | |
| B2H4 | |
| Molar mass | 25.65 g·mol−1 |
| Related compounds | |
Related compounds
|
Bis(pinacolato)diboron Diboron tetrafluoride Tetrahydroxydiborane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Diborane(4) is a transient inorganic compound with the chemical formula B
2H
4. Stable derivatives are known.
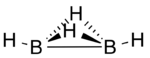
Diborane(4) has been produced by abstraction of two hydrogen atoms from diborane(6) using atomic fluorine and detected by photoionization mass spectrometry. Computational studies predict a structure in which are two hydrogen atoms bridging the two boron atoms via three-centre two-electron bonds in addition to the 2-centre, 2-electron bond between the two boron atoms and one terminal hydrogen atom bonded to each boron atom.
Several stable derivatives of diborane(4) have been reported.
References
- ^ Ruščic, B.; Schwarz, M.; Berkowitz, J. (1989). "Molecular structure and thermal stability of B
2H
4 and B
2H+
4 species". The Journal of Chemical Physics. 91 (8). AIP Publishing: 4576–4581. doi:10.1063/1.456745. - ^ Alkorta, Ibon; Soteras, Ignacio; Elguero, José; Del Beneb, Janet E. (23 June 2011). "The boron–boron single bond in diborane(4) as a non-classical electron donor for hydrogen bonding" (PDF). Physical Chemistry Chemical Physics. 13 (31): 14026–14032. Bibcode:2011PCCP...1314026A. doi:10.1039/C1CP20560A. PMID 21698334.
- ^ Xie, Xiaochen; Haddow, Mairi F.; Mansell, Stephen M.; Norman, Nicholas C.; Russell, Christopher A. (2012). "Diborane(4) compounds with bidentate diamino groups". Dalton Transactions. 41 (7): 2140–7. doi:10.1039/C2DT11936F. PMID 22187045.
- ^ Wagner, Arne; Kaifer, Elisabeth; Himmel, Hans-Jörg (2012). "Diborane(4)–metal bonding: Between hydrogen bridges and frustrated oxidative addition". Chemical Communications. 48 (43): 5277–9. doi:10.1039/C2CC31671D. PMID 22526934.
- ^ Horn, Julian; Widera, Anna; Litters, Sebastian; Kaifer, Elisabeth; Himmel, Hans-Jörg (2018). "The proton affinity, HOMO energy and ionization energy of electron-rich sp3–sp3-hybridized diborane(4) compounds with bridging guanidinate substituents can be varied by substitution". Dalton Trans. 47 (6): 2009–2017. doi:10.1039/C7DT04433J. PMID 29345706.