Flupropadine
In the following article we will explore in depth the topic of Flupropadine, which has captured the attention of experts and enthusiasts alike in recent years. Since its emergence, Flupropadine has generated increasing interest in various sectors, from technology to medicine, and its impact continues to spread globally. Throughout these pages, we will analyze the various aspects related to Flupropadine, from its origin to its possible future implications, with the aim of providing a comprehensive vision of this topic that is so relevant today.
| |
| Names | |
|---|---|
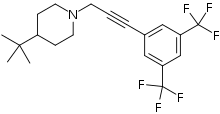
| Preferred IUPAC name
1-{3-prop-2-yn-1-yl}-4-tert-butylpiperidine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H23F6N | |
| Molar mass | 391.401 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Flupropadine is a rodenticide. Originally made by May and Baker and tested on farms in the United Kingdom it was withdrawn from use by 1994. Flupropadine has a delayed action, and so rodents can have multiple feeds from the bait before being killed.
The molecule has two rings, one is a m-hexafluoroxylene, and the other is piperidine. Flupropadine is made from 3,5-bis(trifluoromethyl)iodobenzene, propargyl alcohol, and 4-tert-butylpiperidine.
References
- ^ Buckle, A. P (1985). "Field trials of a new sub-acute rodenticide flupropadine, against wild Norway rats (Rattus norvegicus)". The Journal of Hygiene. 95 (2): 505–12. doi:10.1017/s0022172400062926. PMC 2129537. PMID 3840823.
- ^ Rowe, F. P; Bradfield, A; Swinney, T (1985). "Pen and field trials of flupropadine against the house mouse (Mus musculus L.)". The Journal of Hygiene. 95 (2): 513–8. doi:10.1017/s0022172400062938. PMC 2129553. PMID 4067302.
- ^ Missio, Andrea (14 June 2006). "Hexafluoroxylenes: Fluorine Chemistry and Beyond" (PDF). p. 7.
- ^ Berny, Philipe (May 2003). "STATE-OF-THE-ART REPORT ON THE USE OF ANTICOAGULANT RODENTICIDES IN THE EU AND BEYOND". Communication and Information Resource Centre for Administrations, Businesses and Citizens. Retrieved 15 May 2018.
- ^ Buckle, Alan P.; Smith, Robert H. (2015). Rodent Pests and Their Control, 2nd Edition. CABI. p. 116. ISBN 9781845938178.
- ^ Unger, Thomas A. (1996). Pesticide Synthesis Handbook. William Andrew. pp. 499–500. ISBN 9780815518532.