T-1152
Today, we want to address a topic that is of great importance today: T-1152. Whether due to its impact on society, its historical relevance or its influence in different areas, T-1152 has captured the attention of millions of people around the world. Throughout this article, we will explore different aspects related to T-1152, from its origin to its implications in today's world. We will analyze its importance, its possible consequences and the different perspectives that exist in this regard. It doesn't matter if you are an expert on the subject or are simply curious to learn more about it, this article will give you a detailed and enriching insight into T-1152.
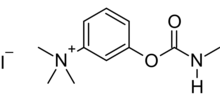
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
270 μg/kg (subcutaneous, mice) 115 μg/kg (intravenous, mice) 260 μg/kg (subcutaneous, rabbits) |
LDLo (lowest published)
|
2.5 mg/kg (oral, mice) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
T-1152 is a quaternary carbamate anticholinesterase. It is synthesized by reaction of m-dimethylaminophenol with methyl isocyanate, followed by quaternization with methyl iodide. Since T-1152 is toxic by ingestion, it was patented as a rodenticide in 1932.
The chloride and methylsulfate salt of T-1152 is T-1690 (TL-1226) and AR-13, respectively.
See also
References
- ^ a b c d Chemical Warfare Agents, and Related Chemical Problems. Parts I-II.
- ^ a b "Product for destroying animals".
- ^ Stedman, Edgar (1 January 1926). "Studies on the Relationship between Chemical Constitution and Physiological Action". Biochemical Journal. 20 (4): 719–734. doi:10.1042/bj0200719. PMC 1251776. PMID 16743713.