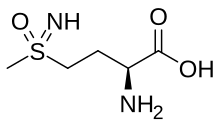
Methionine sulfoximine
This article will address the topic of Methionine sulfoximine, which has been the subject of interest and study over the years. Methionine sulfoximine is a concept that has impacted different areas of daily life, from politics to technology, culture and society in general. Throughout history, Methionine sulfoximine has played a critical role in shaping our perceptions and decision making. Through a detailed analysis, this article aims to explore the different facets of Methionine sulfoximine and its influence in today's world, thus providing a broader and more complete vision of this highly relevant topic.
| |
| Names | |
|---|---|
| IUPAC name
(2S)-2-Amino-4-(S-methylsulfonimidoyl)butanoic acid
| |
| Other names
l-Methionine sulfoximine; MSO
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1725509 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.016.224 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H12N2O3S | |
| Molar mass | 180.22 g·mol−1 |
| Related compounds | |
Related compounds
|
Buthionine sulfoximine Glufosinate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
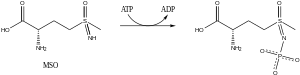
Methionine sulfoximine (MSO, also known as MetSox) is an irreversible glutamine synthetase inhibitor. It is the sulfoximine derivative of methionine with convulsant effects.
Methionine sulfoximine is composed of two different diastereomers, which are L-S-Methionine sulfoximine and L-R-Methionine sulfoximine. These affect the longevity of the model mouse for Lou Gehrig's disease. Overproduction of glutamate results to excitotoxicity, which kills the cell. Since methionine sulfoximine inhibits glutamate production in the brain, it prevents excitotoxicity. Thus, increasing the longevity of the mice.
Mechanism of action
MSO is phosphorylated by glutamine synthetase. The resulting product acts as a transition state analog that is unable to diffuse from the active site, thereby inhibiting the enzyme.
References
- ^ Carroll, P.; Waddell, S. J.; Butcher, P. D.; Parish, T. (2011). "Methionine sulfoximine resistance in Mycobacterium tuberculosis is due to a single nucleotide deletion resulting in increased expression of the major glutamine synthetase, GlnA1". Microbial Drug Resistance. 17 (3): 351–355. doi:10.1089/mdr.2010.0125. PMC 3161625. PMID 21875360.
- ^ Rowe, WB; Meister, A (June 1970). "Identification of L-methionine-S-sulfoximine as the convulsant isomer of methionine sulfoximine". Proceedings of the National Academy of Sciences of the United States of America. 66 (2): 500–6. Bibcode:1970PNAS...66..500R. doi:10.1073/pnas.66.2.500. PMC 283073. PMID 4393740.
- ^ Brusilow, William S. A. (2017-04-24). "Identification of the isomer of methionine sulfoximine that extends the lifespan of the SOD1 G93A mouse". Neuroscience Letters. 647: 165–167. doi:10.1016/j.neulet.2017.03.029. ISSN 0304-3940. PMID 28323087. S2CID 45664203.
- ^ Bame, Monica; Pentiak, Patricia A.; Needleman, Richard; Brusilow, William S. A. (2012-12-01). "Effect of Sex on Lifespan, Disease Progression, and the Response to Methionine Sulfoximine in the SOD1 G93A Mouse Model for ALS". Gender Medicine. 9 (6): 524–535. doi:10.1016/j.genm.2012.10.014. ISSN 1550-8579. PMID 23217569.
- ^ Krajewski, W. W.; Jones, T. A.; Mowbray, S. L. (18 July 2005). "Structure of Mycobacterium tuberculosis glutamine synthetase in complex with a transition-state mimic provides functional insights". Proceedings of the National Academy of Sciences. 102 (30): 10499–10504. Bibcode:2005PNAS..10210499K. doi:10.1073/pnas.0502248102. PMC 1180770. PMID 16027359.