4-Fluorophenibut
In today's world, 4-Fluorophenibut has caught the attention of many people due to its importance and impact on various aspects of life. From its relevance in the educational field to its influence in the workplace, 4-Fluorophenibut has aroused the interest of academics, experts and professionals from different disciplines. In this article, we will explore in detail the different facets of 4-Fluorophenibut, analyzing its evolution over time, its implications in today's society and its projection into the future. Additionally, we will examine the opportunities and challenges that 4-Fluorophenibut represents, as well as the possible implications it has on people's daily lives. We are about to embark on a journey of discovery and reflection about 4-Fluorophenibut, a topic that never ceases to surprise and generate debate in the global community.
 | |
| Clinical data | |
|---|---|
| Other names | CGP-11130; β-(4-Fluorophenyl)-γ-aminobutyric acid; β-(4-Fluorophenyl)-GABA; Baflofen; Fluorophenibut; F-Phenibut; Fluoribut |
| Routes of administration | By mouth |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
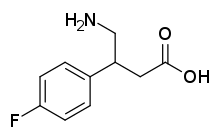
| Formula | C10H12FNO2 |
| Molar mass | 197.209 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
4-Fluorophenibut (developmental code name CGP-11130; also known as β-(4-fluorophenyl)-γ-aminobutyric acid or β-(4-fluorophenyl)-GABA) is a GABAB receptor agonist which was never marketed. It is selective for the GABAB receptor over the GABAA receptor (IC50 = 1.70 μM and > 100 μM, respectively). The drug is a GABA analogue and is closely related to baclofen (β-(4-chlorophenyl)-GABA), tolibut (β-(4-methylphenyl)-GABA), and phenibut (β-phenyl-GABA). It is less potent as a GABAB receptor agonist than baclofen but more potent than phenibut.
The substance is sometimes referred to as 4F-phenibut or F-phenibut or baflofen and colloquially as fluorobut.
Legal status
F-Phenibut is a prohibited substance in Lithuania and Hungary.
References
- ^ a b c d Bowery NG, Hill DR, Hudson AL (1983). "Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes". Br. J. Pharmacol. 78 (1): 191–206. doi:10.1111/j.1476-5381.1983.tb09380.x. PMC 2044790. PMID 6297646.
- ^ "RINKOS RIBOJIMO PRIEMONĖS FENIBUTUI!". ntakd.lrv.lt (in Lithuanian). Retrieved 2020-01-27.
- ^ MAGYARORSZÁG HIVATALOS LAPJA. Retrieved 2021-04-28.